What Are the Two Most Common Findings on Premature Baby Echocardiogram
Abstract
Pulmonary hypertension contributes to morbidity and mortality in both the term newborn babe, referred to as persistent pulmonary hypertension of the newborn (PPHN), and the premature infant, in the setting of abnormal pulmonary vasculature development and arrested growth. In the term infant, PPHN is characterized past the failure of the physiological postnatal decrease in pulmonary vascular resistance that results in impaired oxygenation, right ventricular failure, and pulmonary-to-systemic shunting. The pulmonary vasculature is either maladapted, maldeveloped, or underdeveloped. In the premature baby, the mechanisms are similar in that the early onset pulmonary hypertension (PH) is due to pulmonary vascular immaturity and its underdevelopment, while late onset PH is due to the maladaptation of the pulmonary apportionment that is seen with severe bronchopulmonary dysplasia. This may lead to cor-pulmonale if left undiagnosed and untreated. Neonatologist performed echocardiography (NPE) should exist considered in any preterm or term neonate that presents with chance factors suggesting PPHN. In this review, nosotros discuss the risk factors for PPHN in term and preterm infants, the etiologies, and the pathophysiological mechanisms equally they relate to growth and development of the pulmonary vasculature. Nosotros explore the applications of NPE techniques that assistance in the right diagnostic and pathophysiological assessment of the nigh common neonatal etiologies of PPHN and provide guidelines for using these techniques to optimize the management of the neonate with PPHN.
Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is a complex disorder that is characterized past the presence of an increased pulmonary vascular resistance (PVR) associated with shunting of deoxygenated claret from the pulmonary to the systemic circulation causing severe hypoxemia.
PPHN occurs in about 1–2 per thousand live built-in infants, mostly in term and late preterm newborns.1,2,3,4 It is associated with an increased risk of an agin outcome (five-year survival approximately xc%; neurologic harm in 15–25%) 4,5. The vascular pruning, abnormal vasculature, and vaso-reactivity in infants with bronchopulmonary dysplasia (BPD) ready the tone for the development of pulmonary hypertension (PH) in this population too.half dozen,vii,eight It is a known complication of BPD, with the incidence increasing with the severity of BPD.9 In infants with 'severe' BPD, two recent cohorts put the incidence at >fifty%.9,x Echocardiographic signs of PH in preterm infants in an early on stage (72 h to 14 days of age) are associated with decreased in-infirmary survival and an increased incidence of moderate-severe BPD.11
Common risk factors for the development of PPHN in (almost-) term infants and PH in preterm neonates are summarized in Table 1.7,12,13
The typical clinical flick is a patient with hypoxic cardio-respiratory failure with a pre-/postductal difference in oxygen saturation of ≥five%, although this difference will be attenuated in the presence of a relevant atrial correct-to-left shunt. It should be noted that a pre-/postductal oxygen saturation divergence tin can too be caused by left-sided obstructive centre disease, such as coarctation of aorta, interrupted aortic arch, and hypoplastic left heart disease. Measuring pre- and postductal blood force per unit area can besides exist helpful and may requite insights regarding the presence of shunts. Postductal claret pressure may be heavily influenced by a correct-to-left transductal shunt and falsely miss low preductal blood pressure, which is crucial.
Distinguishing betwixt PPHN and cyanotic heart lesions on clinical grounds can be challenging. This, still, is essential since management for these disorders are quite different. Clinical signs and symptoms suggestive of an underlying congenital middle defect are summarized in Tabular array 2.
Delay in the diagnosis and appropriate treatment of a cyanotic centre disease is associated with worsening prognosis. It is therefore imperative to accept a comprehensive echocardiographic evaluation to rule out any structural abnormality. When in uncertainty during the time earlier echocardiography tin can be performed, it is advised to get-go prostaglandin E1 in apprehension of a potential centre defect. In a patient with PPHN, prostaglandin E1 might have some pulmonary vasodilatory furnishings, and in severe PPHN, information technology will preserve postductal systemic perfusion, albeit at the expense of cyanosis. It should exist noted that the institution of a handling regimen aiming at pulmonary vasodilation may deteriorate the clinical status of the patients with certain heart defects, such every bit total anomalous pulmonary venous connectedness (TAPVC) and hypoplastic left centre syndrome.
The following structural heart defects may clinically mimic PPHN: TAPVC, transposition of keen arteries (TGA), pulmonary atresia with or without ventricular septal defect (VSD), severe Fallot'south tetralogy, tricuspid atresia, unguarded tricuspid orifice syndrome, severe Ebstein anomaly, and sometimes fifty-fifty left-sided obstructive heart affliction (such as coarctation of aorta, interrupted aortic arch, and hypoplastic left heart affliction).
Etiology
Pulmonary avenue pressure (PAP) is adamant by pulmonary claret menses (PBF), PVR, and pulmonary capillary wedge pressure (PcWP), as shown in the following Eq. i.
$$\hskip 70pt{\mathrm{PAP}}\,{\mathrm{ = }}\,{\mathrm{PcWP}}\,{\mathrm{ + }}\,\left( {{\mathrm{PBF}}\,\,\times\,{\mathrm{PVR}}} \right).$$
(1)
Under normal circumstances, PAP falls after birth within ii months to achieve a level that is comparable to adult values (systolic PAP <25 mmHg).
As can be derived from Eq. i, PH can exist caused by an increase in PcWP (left heart failure, for example secondary to arteriovenous malformations, such as vein of Galen aneurysmal malformation (VGAM)), past an increase in PBF (e.g., large left-to-right shunt with pulmonary hyper-perfusion), or by raised PVR (east.yard., pulmonary vasoconstriction). A combination of these factors is likewise possible.
In PPHN, the rise in pulmonary claret pressure is by and large secondary to an increased PVR with the following etiology:14
- 1.
Maladaption of pulmonary vasculature (abnormal, 'reactive' pulmonary vasoconstriction)
- 1.1.
due to parenchymal lung diseases, such equally meconium aspiration syndrome (MAS), respiratory distress syndrome (RDS), hypoventilation, and pneumonia
- 1.ii.
in response to sure stimuli, such as hypothermia, sepsis, stress, hypercapnia, hypoxemia, acidosis, and hyperviscosity
- i.3.
toxic/pharmacological (maternal SSRI apply)
- 1.1.
- ii.
Maldevelopment of pulmonary vasculature (remodeling of pulmonary vasculature) in response to:
- ii.1.
in utero closure of ductus arteriosus (for example maternal cyclooxygenase inhibitor use)
- 2.ii.
pulmonary hyperperfusion in congenital heart illness with large left-to-right shunt
- 2.3.
infants with fetal growth brake
- ii.1.
- 3.
Underdevelopment of pulmonary vasculature (hypoplastic pulmonary vessels; decreased cantankerous-sectional area), such equally in:
- 3.ane.
congenital diaphragmatic hernia
- 3.ii.
pulmonary hypoplasia (premature prolonged rupture of membranes, oligohydramnios and anhydramnios).
- 3.ane.
Respiratory disorders that are associated with PH include: congenital diaphragmatic hernia, BPD, alveolar capillary dysplasia (with or without misalignment of veins), lung hypoplasia ('master' or 'secondary'), surfactant protein abnormalities, pulmonary interstitial glycogenosis, pulmonary alveolar proteinosis, and pulmonary lymphangiectasis.15 Recently, it was recognized that PH tin exist acquired by pulmonary venous stenosis, especially in preterm infants with BPD, that is often overlooked.16,17 In about ten–20%, no specific cause of PPHN is found ('idiopathic' PPHN).xiv
Hemodynamic profile of pphn
The key features in PPHN are an increased PVR resulting in high PAP, ductal and/or atrial right-to-left shunting, and correct (and ultimately left) ventricular dysfunction. This leads to:
-
• Right ventricular systolic and diastolic failure secondary to increased afterload
-
• Decrease in RV stroke volume and RV filling
-
• Decrease in pulmonary blood menses with ventilation-perfusion mismatch
-
• RV dilatation causing a D-shaped left ventricle with decreased LV preload
-
• Decreased LV stroke volume
-
• Right-to-left shunting through the ductus arteriosus and/or foramen ovale.
In a severe course of PPHN, this ductal and/or atrial right-to-left shunt may guarantee postductal or preductal systemic perfusion, respectively, all the same, at the expense of cyanosis.
Echocardiography
Comprehensive echocardiography is indicated when there is a clinical suspicion of PPHN to exclude congenital eye disease.
Neonatologist performed echocardiography (NPE) is useful in multiple ways: (a) making the diagnosis and grading the severity, (b) determining the demand for specific (pulmonary vasodilator) or supportive (choice of inotrope) therapy, (c) monitoring the response to therapy, and (d) rational weaning of therapy. This is especially relevant when administering inhaled nitric oxide (iNO) for infants <34 weeks' gestation, where multiple randomized controlled trials accept suggested limited evidence.18,19 Co-ordinate to the recent AHA/ATS guidelines, iNO tin be beneficial for preterm infants with astringent hypoxemia that is due primarily to PPHN physiology rather than parenchymal lung disease, particularly if associated with prolonged rupture of membranes and oligohydramnios.20
Once the diagnosis of PPHN is confirmed past echocardiography, the clinical form and the effects of medical interventions can be monitored using NPE with the accent on:
1. pulmonary artery pressure level and PVR,
2. myocardial operation, and
3. shunting through ductus arteriosus and open foramen ovale.
An overview of all echocardiographic parameters that tin exist assessed with NPE is presented in Tabular array 3.
Estimation of PAP or PVR
PAP can be assessed by measuring tricuspid valve regurgitation peak velocity, pulmonary regurgitation elevation velocity, transductal right-to-left flow peak velocity, interventricular septum (IVS) configuration, and LV systolic eccentricity alphabetize (LV-sEI).
Tricuspid regurgitation elevation velocity
Systolic pulmonary artery pressure (SPAP) tin be estimated by measuring the peak velocity of tricuspid valve regurgitation with the use of the modified Bernoulli's equation; see Eqs. 2 and 3:
$$\begin{assortment}{50}p = 4\,\times\,{5}^two\\ \quad \,\hskip -14pt\left({p,{\rm{pressure}}\,{\rm{gradient}}\ \left( {{\rm{in}}\,{\rm{mmHg}}} \right);\,v,{\rm{blood}}\,{\rm{velocity}}\,\left({{\rm{in}}\,{\rm{m}}/{\rm{due south}}} \correct)} \correct),\stop{assortment}$$
(two)
$$\brainstorm{array}{l}{\mathrm{SPAP}} \approx {\mathrm{RVSP}} = {\mathrm{4}}\,\times\left( {{\mathrm{VmaxTR}}} \correct)^{\mathrm{2}} + {\mathrm{RAP}}\\ \quad \left( {{\rm{RSVP}},{\rm{right}}\,{\rm{ventricular}}\,{\rm{systolic}}\,{\rm{pressure}}\,\left({{\rm{in}}\,{\rm{mmHg}}}\right);} \right.\\ \quad {\rm{VmaxTR}},{\rm{peak}}\,{\rm{velocity}}\,{\rm{of}}\,{\rm{tricuspid}}\,{\rm{regurgitation}}\,\left({{\rm{in}}\,{\rm{m}}{\mathrm{/}}{\rm{southward}}} \right);\\ \quad \left. {{\rm{RAP}},{\rm{right}}\,{\rm{atrial}}\,{\rm{pressure level}}\,\left( {{\rm{in}}\,{\rm{mmHg}}}\right)}\right)\end{array}$$
(three)
RAP is usually not measured, and a value of three–5 mmHg is generally assumed. The estimation of SPAP by measuring TR is reliable and often equivalent to pressures measured in the catheter lab while using continuous wave Doppler (Fig. ane). However, the accuracy depends on the quality of the acquired TR jet. An optimal quality TR jet shows a well demarcated envelope. Measuring an inadequate Doppler spectral envelope will potentially lead to an underestimation of SPAP. The angle of insonation should be less than 20° to attain a reliable measurement. This is aided past assessing maximal TR jet velocity past imaging from three views (apical 4 chamber, short axis, and modified parasternal long centrality). Notwithstanding, this interpretation of SPAP is non reliable in the presence of right ventricular failure or correct ventricular outflow tract obstruction. Tricuspid valve regurgitation cannot ever be observed and is present in approximately lx–85% of patients with PPHN.21,22,23,24,25
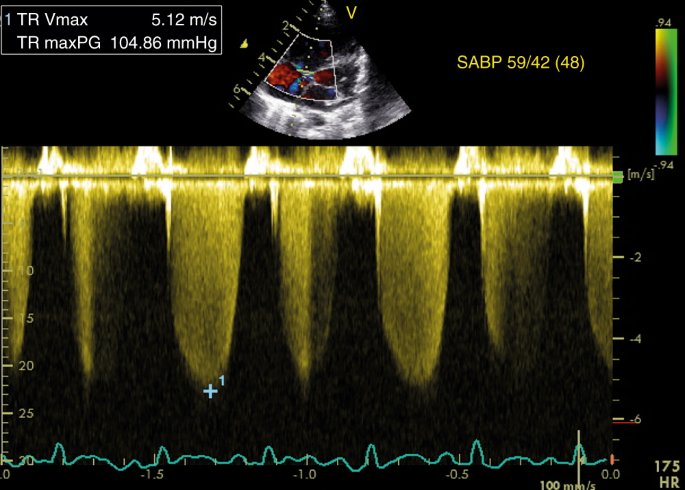
Estimation of SPAP from tricuspid regurgitation jet. The peak velocity of the tricuspid regurgitation jet is v.12 k/s, which corresponds to a maximum pressure gradient of 105 mmHg according to the modified Bernoulli equation (encounter left upper console). On the correct, the concomitant systemic arterial blood pressure is displayed, indicating suprasystemic pulmonary pressure level
Pulmonary regurgitation meridian velocity
In the presence of pulmonary valve regurgitation, mean PAP (MPAP) can be estimated past measuring its peak velocity using Eq. four:
$$\begin{array}{l}{\mathrm{MPAP}} = {\mathrm{4}}\,\times\left( {{\mathrm{VmaxPR}}} \right)^{\mathrm{ii}} +\, {\mathrm{RVdP}}\\ \quad \left( {{\mathrm{MPAP}},{\mathrm{mean}}\,{\mathrm{pulmonary}}\,{\mathrm{artery}}\,{\mathrm{pressure}}\,\left( {{\mathrm{in}}\,{\mathrm{mmHg}}} \right);} \right.\\ \quad {\mathrm{VmaxPR}},{\mathrm{meridian}}\,{\mathrm{velocity}}\,{\mathrm{pulmonary}}\,{\mathrm{valve}}\,{\mathrm{incompetence}}\,\left( {{\mathrm{in}}\,{\mathrm{m}}{\mathrm{/}}{\mathrm{due south}}} \right);\\ \quad \left. {{\mathrm{RVdP}},\,{\mathrm{correct}}\,{\mathrm{ventricular}}\,{\mathrm{diastolic}}\,{\mathrm{pressure}}\,\left( {{\mathrm{in}}\,{\mathrm{mmHg}}} \right)} \right).\end{array}$$
(four)
The RVdP is generally causeless to be around 2–5 mmHg.
Transductal right-to-left blood flow peak velocity
Transductal correct-to-left claret flow tin can be used to approximate SPAP, when it lasts ≥thirty% of the heart wheel, by measuring its meridian velocity using Eq. 5:
$$\begin{assortment}{50}{\mathrm{SPAP}} = {\mathrm{four}}\,{\mathrm{x}}\,\left( {{\mathrm{VmaxDA}}} \right)^{\mathrm{2}}\, + \,{\mathrm{SSAP}}\\ \quad \left( {{\mathrm{SPAP}},{\mathrm{systolic}}\,{\mathrm{pulmonary}}\,{\mathrm{artery}}\,{\mathrm{pressure}}\,\left( {{\mathrm{in}}\,{\mathrm{mmHg}}} \correct);} \right.\\ \hskip -14pt\quad {\mathrm{VmaxDA}},{\mathrm{peak}}\,{\mathrm{velocity}}\,{\mathrm{ductal}}\,{\mathrm{correct}}{\mathrm{ - }}{\mathrm{to}}{\mathrm{ - }}{\mathrm{left}}\,{\mathrm{shunt}}\,\left( {{\mathrm{in}}\,{\mathrm{m}}{\mathrm{/}}{\mathrm{s}}} \correct);\\ \quad \left. {{\mathrm{SSAP}},{\mathrm{systolic}}\,{\mathrm{systemic}}\,{\mathrm{arterial}}\,{\mathrm{pressure}}\,\left( {{\mathrm{in}}\,{\mathrm{mmHg}}} \correct)} \right).\end{assortment}$$
(5)
A ductal right-to-left or bidirectional shunt is observed in 73–91% of the patients with PPHN.11,12,15 However, measurement of PAP via ductal period is oftentimes not reliable. Cess of the direction of transductal blood menses is more useful and will indicate the relation between pulmonary and systemic pressures.
IVS configuration/LV-sEI
An culling, although more subjective, estimation of PAP is based upon the alignment of the IVS (Table four). Normally the septum bows into the correct ventricle (O-shaped LV) and with increasing right ventricular force per unit area, the IVS will flatten (D-shaped LV) and eventually curves into the left ventricle (crescent-shaped LV) (meet Fig. 2).v It is best analyzed at the end of systole in the parasternal brusk axis view above the level of the papillary muscles.26
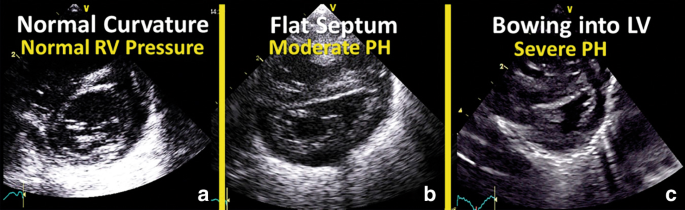
Morphology of interventricular septum. Commonly the septum bows into the right ventricle (a; O-shaped LV) and with increasing right ventricular pressure, the interventricular septum will flatten (b; D-shaped LV) and somewhen curves into the left ventricle (c; crescent-shaped LV)
A more objective estimation is made past computing the LV-sEI, which is the ratio of LV dimension parallel and perpendicular to the septum, respectively. Figure 3 shows the measurement of LV-sEI from brusque axis parasternal view. The LV-sEI measure is a derivation from IVS configuration. Normal LV-sEI ratio is typically 1 and equally it increases in PH, information technology allows for quantification of a more subjective parameter of IVS flattening/bowing. In adult literature, a ratio >i in systole denotes RV pressure overload. In a recent written report on infants with BPD associated PH, LV-sEI was significantly higher in the infants with PH.27 LV diastolic EI is more than a mark of volume overloaded right ventricle; for clinical atmospheric condition such as PPHN, pressure overload predominates, and hence the usefulness of the sEI.
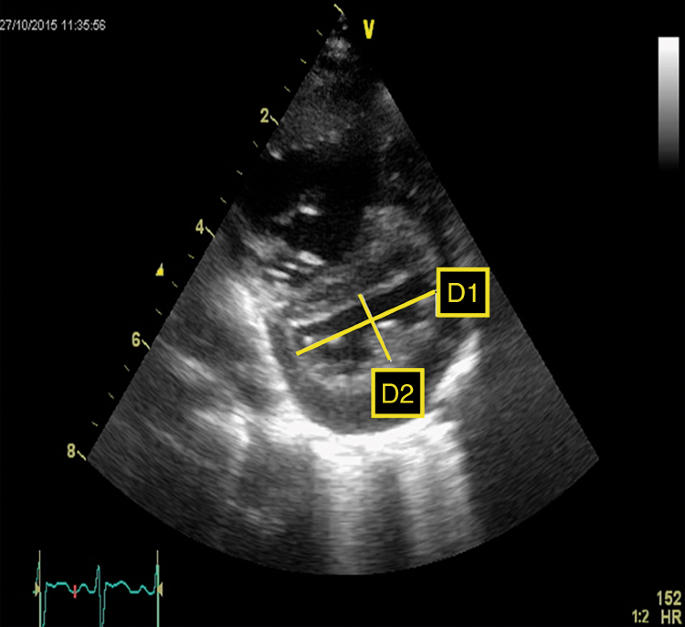
LV systolic eccentricity index (LV-sEI). Left ventricle from brusque axis view showing flattened septum and high systolic eccentricity alphabetize (LV-sEI =Di/D2)
PVR tin can be assessed past measuring correct ventricular systolic time intervals (pulmonary artery acceleration time (PAAT), right ventricular ejection time (RVET), correct ventricular pre-ejection time (RVPET)), TRV:VTI[RVOT]-ratio (ratio between tricuspid regurgitation velocity (TRV) and the velocity–time integral (VTI) of claret flow through the right ventricular outflow tract (RVOT)), and pulmonary artery compliance.
Right ventricular systolic time intervals
Right ventricular systolic time intervals are another validated method for the estimation of PVR. The following right ventricular time intervals can be derived from the Doppler PBF velocity curve: RVET, RVPET, and PAAT, also referred to every bit time to peak velocity (TPV) (see Fig. four).
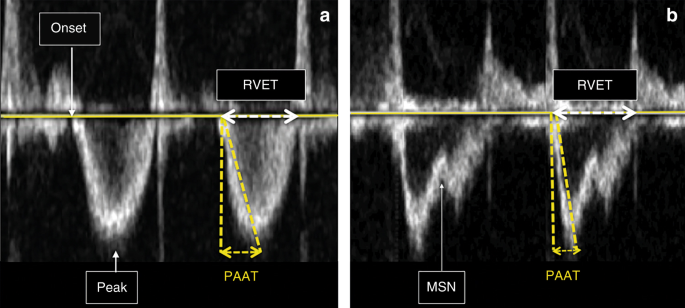
Right ventricular systolic time intervals. a Normal pulmonary avenue pressure/pulmonary vascular resistance. b Increased pulmonary artery pressure/pulmonary vascular resistance (MSN mid-systolic notch, PAAT pulmonary artery dispatch fourth dimension, top peak velocity, RVET right ventricular ejection time). Encounter text for details
Until recently, the methods for the interpretation of PAP using the summit velocity of pulmonary regurgitation was considered more than reliable than right ventricular systolic time intervals, since the repeatability of these time intervals was shown to be rather disappointing.28,29,xxx However, PAAT has recently been validated as a feasible and reproducible, non-invasive echocardiographic imaging marker, for detection of pulmonary vascular illness and PH in neonates and children.31 This study, with simultaneous Doppler echocardiography and invasive catheterization, established PAAT-based regression equations in children to accurately predict invasive catheterization-derived SPAP and PVR. A cutoff value of <xc ms reliably detects pulmonary vascular disease, and a value <40 ms detects pulmonary vascular illness in its most severe class of PH. The normal value of the PAAT:RVET ratio is approximately 0.31 or greater. A PAAT:RVET ratio less than 0.23 and/or an increased RVPET:RVET ratio is indicative for increased PAP.31 Visual inspection of the shape of the Doppler catamenia envelope pattern across the RV outflow tract is a sensitive predictor of PH and right centre dysfunction in children and infants. The mid-systolic notch, besides referred to every bit the "flying W", is associated with elevated PVR and PAP (Fig. 4—right panel).32
TRV/VTI[RVOT]
PVR can be estimated past calculating the TRV:VTI[RVOT] ratio, which is the ratio between TRV and the VTI of blood flow through the RVOT using pulsed-moving ridge Doppler in the parasternal short axis.33,34,35 The obtained VTI in the RVOT volition be markedly changed in the presence of loftier PVR due to an earlier and enhanced reflection of the force per unit area wave. College PVR will atomic number 82 to a subtract in VTI[RVOT]. TRV:VTI[RVOT] ratio have been shown to correlate well with PVR in children and a cut off value of 0.xiv provided high predictive values.34 Nevertheless, neonatal studies are lacking.
Pulmonary artery compliance
Echocardiography can be used to guess dynamic pulmonary avenue compliance (CdynPA) non-invasively by measuring pulmonary bore in systole (Ds) and diastole (Dd) and analyzing the TR jet to calculate SPAP.36
$$\begin{array}{50}{\mathrm{CdynPA}} = \left[ {\left( {{\mathrm{Ds}} - {\mathrm{Dd}}} \right){\mathrm{/}}\left( {{\mathrm{Dd}} \times {\mathrm{SPAP}}} \correct)} \right] \times {\mathrm{x}}^{\mathrm{4}}\\ {\mathrm{CdynPA}},{\mathrm{dynamic}}\,{\mathrm{pulmonary}}\,{\mathrm{artery}}\,{\mathrm{compliance}} \\ \left( {{\mathrm{in}}\,\% \,{\mathrm{change}}{\mathrm{/}}100\,{\mathrm{mmHg}}} \right) D,{\mathrm{diameter}}\,\left( {{\mathrm{in}}\,{\mathrm{cm}}} \right).\end{array}$$
(6)
Lower CdynPA is found in children with PH.36 There is a paucity of data on this measurement in the term and preterm neonatal population.
Qualitative assessment of right ventricular, right atrial, and pulmonary diameters
An increased PVR can cause an increased pulmonary artery diameter. Impaired right ventricular systolic and diastolic performance will lead to dilation of the right ventricle, right atrium, and inferior vena cava. Normative data for right ventricular size have been published.37,38,39,40
Myocardial operation
Biventricular dysfunction tin can be plant in up to 70% of patients with PPHN and is thought to be related to increased right ventricular afterload, decreased left ventricular preload in addition to possible myocardial ischemia.24,25
Classical PPHN (acute, soon afterwards nativity, secondary to pathologies like MAS) is a pre-capillary disorder. The right center is dilated but the LV is not (due to reduced preload to LV, and septal shift into the LV). One might observe lower PcWP, as the malady is caused past elevated PVR. Hence, pulmonary vasodilators work well. BPD associated PH is another beast, and some infants have post-capillary pathophysiology as an important correspondent. This group is clinically characterized by an elevated PcWP and no response to or deterioration subsequently commencement of pulmonary vasodilators such as iNO and sildenafil. Unfortunately, invasive cardiac catheterization is not hands available, and many of these infants may not tolerate that. Echocardiographic clues to this malady are dilated LV and increased LA end-diastolic force per unit area. This cohort needs systemic and not pulmonary vasodilators as the pathophysiology centers on elevated 'systemic' more than than 'pulmonary afterload'.41
A nigh consequent finding in (near-)term patients with PPHN is a low left ventricular output (LVO) and reduced LV stroke volume (LV-SV) in combination with normal or mildly decreased LV-EF, that is explained by reduced preload secondary to right-to-left shunting (decreased pulmonary venous return) and ventricular–ventricular interaction (flattening of IVS).21,22,25
Echocardiographic markers of left ventricular failure (reduced LV size and stroke volume) are associated with the need for more than intense treatment, such every bit high frequency ventilation and ECMO in newborn infants diagnosed with PPHN.23
Right ventricular office can be assessed by measuring the fractional area change (FAC), myocardial performance alphabetize (RV-MPI), right ventricular systolic to diastolic duration ratio (RV South/D ratio), and tricuspid annular airplane systolic excursion (TAPSE).
Left ventricular function tin can be analyzed by monitoring myocardial performance (LV-MPI), LV-SV and LVO, and ejection fraction (EF biplane Simpson).
By using tissue Doppler imaging (TDI) and speckle-tracking echocardiography (STE), the performance of both ventricles can exist assessed.
Tricuspid annular airplane systolic circuit (TAPSE)
TAPSE is a measure of RV longitudinal function and obtained from the iv-sleeping room view using the M-Mode with the cursor aligned along the management of the lateral annulus (see Fig. v). TAPSE provides useful information about longitudinal fiber shortening and information technology has shown skillful correlation with techniques estimating RV global systolic office. All the same, it should be noted that TAPSE is both angle and load dependent. Normal values in the neonatal population tin can be obtained from Koestenberger et al.42 Diminished TAPSE (<4 mm) is predictive for the need of ECMO and expiry in infants with PPHN.43
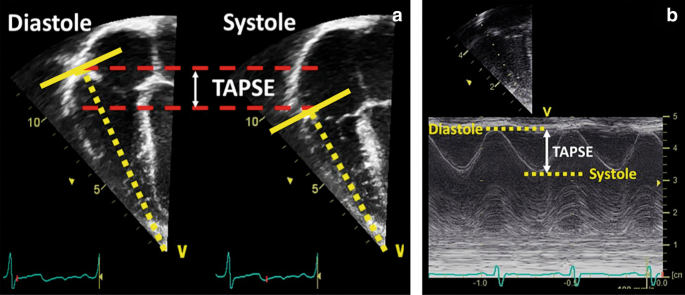
Tricuspid annular airplane systolic excursion (TAPSE). TAPSE is a measure of RV longitudinal function and obtained from the 4-chamber view using the G-Mode with the cursor aligned along the direction of the lateral annulus. The traveled distance of the tricuspid annulus from diastole to systole is expressed in millimeters
Fractional area alter (FAC)
FAC is a planimetric measure of the ratio of systolic to diastolic expanse in upmost 4- or iii-chamber view by manual tracing of the endocardial border of the right ventricle. Different TAPSE, right ventricular FAC is afflicted past radial, basal, and apical functions equally well as longitudinal cobweb shortening, but it is considered to be more prone to operator-dependent variation. FAC is calculated by the following formula:
$${\mathrm{FAC}} = \left[ {{\mathrm{RV}}\,{\mathrm{area}}\,\left( {{\mathrm{diastole}}} \correct)-{\mathrm{RV}}\,{\mathrm{area}}\,\left( {{\mathrm{systole}}} \correct)} \right]{\mathrm{/RV}}\,{\mathrm{area}}\,\left( {{\mathrm{diastole}}} \right).$$
(vii)
It is important that the entire ventricle is visualized when tracing the endocardium in systole and diastole including the outflow tract and the lateral wall (Fig. 6). Trabeculation should exist included within the cavity under the tracing procedure. Normal values (25–45%) in preterm and term infants have been published.38,43,44 Median values of 19% were associated with the need for ECMO or death.43
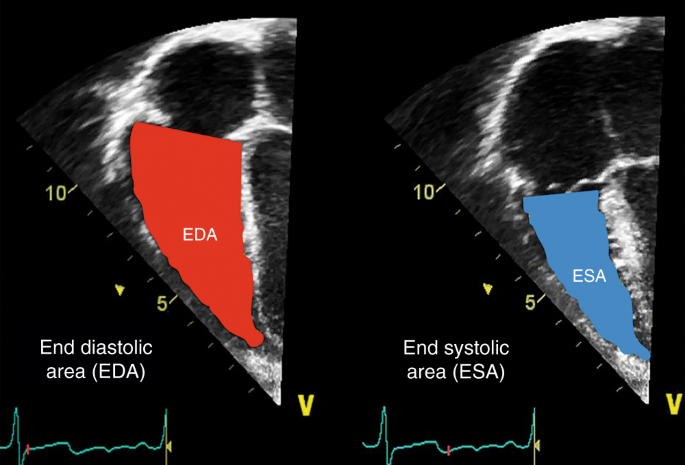
Partial area change (FAC). FAC is a planimetric measure out of the ratio of finish systolic area (ESA) to end diastolic area (EDA) in upmost 4- or 3-chamber view by transmission tracing of the endocardial border of the right ventricle (FAC (%) = [EDA−ESA]/EDA). Trabeculation should be included within the cavity nether the tracing procedure
Myocardial Performance Alphabetize (MPI)
The MPI, likewise referred to as Tei index, represents the relation betwixt the sum of isovolumic contraction and relaxation time and ejection fourth dimension and can be derived from pulsed Doppler or tissue Doppler (Fig. 7). Right ventricular dysfunction (simply too increased RV afterload) will increase the fourth dimension of isovolumic phases and therefore lead to a higher MPI. The index is usually used to estimate global ventricular function of the left ventricle, but the awarding in the pediatric and neonatal population for right ventricular performance is widely accepted.45,46 MPI of both the RV and LV are significantly elevated in infants with PPHN.25
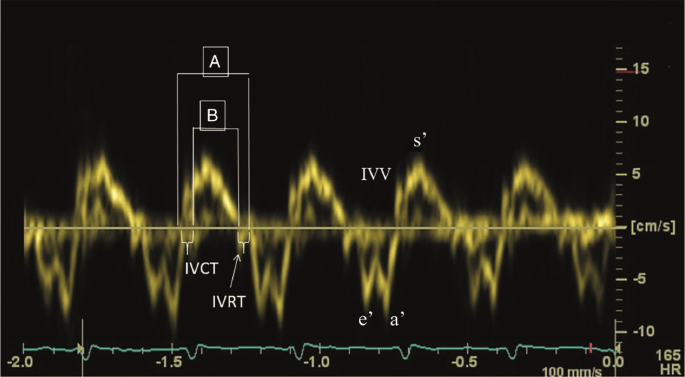
Myocardial Performance Index—MPI or Tei index. Pulse wave Tissue Doppler waveform (isovolumic wrinkle time (IVCT), isovolumic relaxation time (IVRT), peak isovolumic systolic (IVV), early diastolic (due east′), late diastolic (a′), elevation systolic (s′) velocity). Myocardial performance alphabetize = the sum of isovolumic contraction and relaxation fourth dimension divided past ejection time (A−B/A)
Global RV myocardial performance index (MPI) can subsequently be calculated using Eq. viii .
Reference values for these parameters in the neonatal menstruum have been published recently.38,42,47
$${\mathrm{MPI}} = \left( {{\mathrm{IVET}} + {\mathrm{IVRT}}} \correct){\mathrm{/RVET}} \hfill\\ \left( {{\mathrm{IVET}},{\mathrm{isovolumic}}\,{\mathrm{ejection}}\,{\mathrm{fourth dimension}};} \correct. \hfill \\ {\mathrm{IVRT}},\,{\mathrm{isovolumic}}\,{\mathrm{relaxation}}\,{\mathrm{time}}; \hfill\\ \left. {{\mathrm{RVET}},{\mathrm{right}}\,{\mathrm{ventricular}}\,{\mathrm{ejection}}\,{\mathrm{time}}} \right).$$
(viii)
Right ventricular systolic to diastolic elapsing ratio (RV South/D ratio)
The right ventricular systolic to diastolic duration ratio (RV S/D ratio) is an index of systolic and diastolic (global) function of the right ventricle and is assumed to reverberate ventricular loading and contractility. An increment in Due south/D ratio is seen equally a sign of global right ventricular dysfunction secondary to increased afterload.
The Southward/D ratio is calculated from the Doppler indicate of tricuspid valve regurgitation. The duration from onset to termination of tricuspid valve regurgitation is the systolic elapsing (SD) and the diastolic duration is the time between two jets of tricuspid regurgitation (DD), see Fig. 8.48 The RV South/D ratio is related to SPAP and RV performance.24,25,48 An increased RV Due south/D ratio (>ane.iii) is associated with the need for ECMO or decease.25
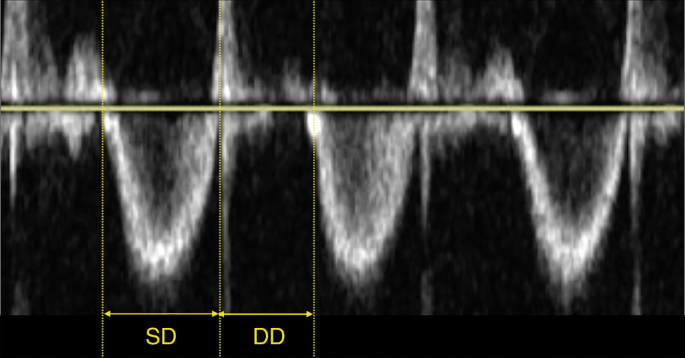
The right ventricular systolic to diastolic duration ratio (RV S/D ratio) is an index of systolic and diastolic (global) function of the right ventricle and is assumed to reflect ventricular loading and contractility (SD systolic duration, DD diastolic duration)
Tissue Doppler Imaging (TDI)
Diastolic part is traditionally studied by measuring the tricuspid valve inflow velocities (Early (East), Belatedly (A), and ratio (E/A)) during diastole past Pow Doppler from the upmost 4-chamber view.
TDI derived deformation imaging calculates strain rate by assessing the deviation in velocity (the velocity slope) between two points along the longitudinal aeroplane of the iv-bedroom view. Strain is then derived by integrating time into the strain charge per unit values. Only deformation along (parallel to) the beam of the ultrasound is measured past the TD method and is therefore highly dependent on the angle of insonation.
TDI of the right ventricular lateral wall with the sampling gate positioned at the junction of tricuspid annulus allows assessment of systolic and diastolic velocities.
Meridian systolic (s′), early and late diastolic (e′ and a′) myocardial velocities tin be hands obtained by TDI, including time periods of closing to opening of the tricuspid valve (TcOT′), isovolumic relaxation time (IVRT), and the elapsing of systole (Southward) and diastole (D) with the resultant Southward/D ratio.
Reduced systolic and diastolic TDI velocities have been found in neonates with PH.49 In addition, reduced early diastolic velocity on days 1 and two of life predicted early on respiratory result in infants with congenital diaphragmatic hernia.50
In term infants with astringent PPHN not responsive to iNO, RV strain (−17%) and strain rates (−1.5 1/due south) significantly improves following the administration of milrinone over a 24-h flow (to −23% and −ii.2 1/s, respectively).51 This further highlights the ability of deformation parameters to identify myocardial dysfunction and monitor handling response.
Speckle-tracking echocardiography (STE)
STE has been successfully used in the estimation of right ventricular office in term and preterm infants, although the technique was originally adult and validated for the left ventricle of adults.38,52,53 Equally the longitudinal shortening is the master deformation of the right ventricle, longitudinal strain seems to be the most robust parameter in describing systolic right ventricular function.52 In improver, diastolic measurements of early on and tardily myocardial movements tin exist obtained. As an angle-independent method, it does not require geometric assumptions and has been used in the neonatal and preterm population. In term infants with PPHN, a reduced global systolic height strain of the RV was associated with progression to death or ECMO.43 In comparison to healthy controls, term infants with PPHN in the first week of age accept worse RV function as shown by a subtract in the magnitude of RV global longitudinal strain.54 Similarly, preterm infants with belatedly onset PH (~36 weeks postmenstrual age) also displayed lower values of RV global and gratis wall longitudinal strain when compared to preterm infants without PH.55 Sehgal et al. recently reported the case of a three-month-sometime infant where RV part was monitored using STE in response to iNO administration.56 A sequential change in global and segmental strain was observed and regional asynchrony in segmental deformation was noted additionally in response to iNO administration. Basal and centre lateral segments showed paradoxical strain (lengthening, positive value). Cess of regional RV function helped understand adaptive mechanisms and assess therapeutic interventions.
Ductal and/or atrial shunting
Secondary to increased pulmonary arterial pressure, deoxygenated claret can shunt through the fetal channels (ductus arteriosus and foramen ovale) to the systemic circulation leading to hypoxemia (Fig. 9).
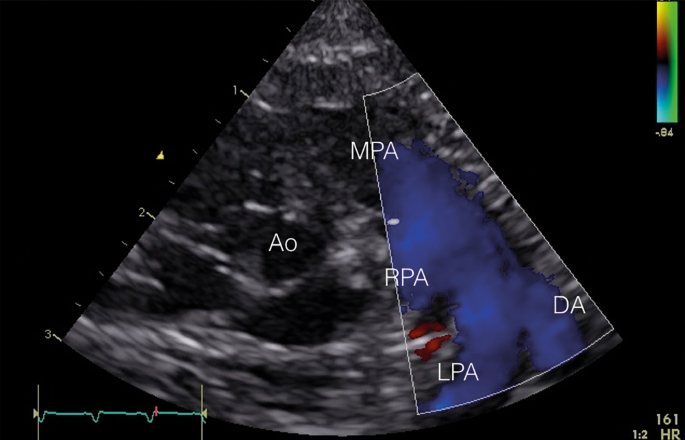
Transductal right-to-left shunting. In the parasternal curt axis view, the primary pulmonary avenue (MPA) is shown with the right pulmonary artery (RPA), the left pulmonary artery (LPA), and the ductus arteriosus (DA) with a clear right-to-left shunt (blueish in color Doppler)
In 73–91% of patients with PPHN, a transductal right-to-left or bidirectional shunt can be observed.21,22,25 A bidirectional shunt with a systolic right-to-left elapsing ≥30% of the full heart bicycle is considered not-physiologic and probable to represent PPHN. An atrial bidirectional or correct-to-left shunt is detected in 73–100% of PPHN patients.21,22,25 Also, left-to-right shunting over the interatrial septum is possible, since in PPHN diastolic pulmonary avenue force per unit area is generally sub-systemic with suprasystemic SPAP. A pure right-to-left shunt at the atrial level suggests TAPVC until proven otherwise.
An exclusive right-to-left transductal shunt in patients with PPHN is associated with an increased risk of mortality.22 However, this does not imply that shunting is the primary problem; information technology is simply a marker of disease severity. In the presence of RV dysfunction, shunting through the fetal channels is a mechanism to augment systemic claret flow and offset high RV afterload. The PFO functions as the modulator of (preductal) cerebral claret menses, whereas the PDA modulates blood menses to the torso and to some extent to the brain.
Practical echocardiographic approach to pphn
1 of the most of import uses of echocardiography in the NICU is in the management of PPHN. Echocardiography should be performed to rule out a congenital eye defect, diagnose PPHN, assess myocardial role, and guide therapy (fluid bolus, option of cardiovascular drugs). Serial echocardiography is useful in monitoring the response to the treatment in PPHN. Nosotros advise a guidance on the assessment of PPHN using NPE, as depicted below. This list is non exhaustive, although not all patients would need all the measurements in the management of PPHN.
- 1.
Structural assessment of the heart to rule out any congenital middle defect, especially duct-dependent center conditions or significant congenital middle defects; should exist done on the first echocardiogram or at the earliest opportunity in a sick neonate.
- 2.
Assessment of (systolic) PAP—this can exist measured accurately if there is tricuspid regurgitation (TR). Absence of TR or minimal TR does non rule out PPHN. Cess of PAP cannot be reliably done via ductal or atrial shunt. All the same, management of the shunt will give a good indication virtually the pressure in relation to the systemic claret pressure.
- 3.
Cess of the shunt direction across ductus arteriosus—right to left, left to right, or bidirectional
- 4.
Assessment of shunt across foramen ovale—correct to left, left to right, or bidirectional. If atrial shunt is purely right to left it should be considered secondary to TAPVC until proven otherwise.
- v.
Assessment of IVS morphology—flattening or bowing of IVS towards LV advise supra-systemic PAP or significant book overloading of the right ventricle (failing right ventricle)
- 6.
Correct ventricular size—dilated or hypertrophied or both. RV, RA, and pulmonary artery are commonly enlarged.
- 7.
Objective assessment of RV function (by the methods described in the text—TAPSE, TDI of IVS and RV free wall, PAAT/RVET ratio, RV South/D ratio, RV partial change, or STE) is recommended. Subjective assessment of RV performance is unreliable and inaccurate.
- 8.
Trend of VTI across pulmonary valve or correct ventricular output can be used in monitoring the progress without estimating the cardiac output. The cardiac output is oft contaminated past the shunts in this population.
- ix.
Objective cess of LV part (monitoring myocardial performance (LV-MPI), LV-SV and LVO, ejection fraction (EF biplane Simpson), TDI of IVS and LV gratuitous wall, or STE) is recommended.
- 10.
Focused serial echocardiography should be performed to observe the progression, especially in sick infants who are non responding to the intervention.
Conclusion
PH is a serious disorder that may occur in both term and preterm infants. Neonatologist performed echocardiography is very useful for the timely diagnosis of PH and targeting handling to forestall morbidity and bloodshed. For these purposes, many echocardiographic variables can be evaluated, but unfortunately not one of them is the ultimate predictive parameter to assess and manage PH. It is advised to ever perform a comprehensive echocardiographic examination. One should bear in listen that NPE volition not improve outcome on its own, but information technology is used to guide treatment and monitor hemodynamic responses that might prove benign for the patient's prognosis.
References
-
Walsh-Sukys, M. C. et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 105(1 Pt 1), 14–20 (2000).
-
Lipkin, P. H. et al. Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J. Pediatr. 140, 306–310 (2002).
-
Hosono, S. et al. Developmental outcomes in persistent pulmonary hypertension treated with nitric oxide therapy. Pediatr. Int. 51, 79–83 (2009).
-
Jain, A. & McNamara, P. J. Persistent pulmonary hypertension of the newborn: advances in diagnosis and treatment. Semin. Fetal Neonatal Med. xx, 262–271 (2015).
-
Bendapudi, P., Rao, G. Thou. & Greenough, A. Diagnosis and management of persistent pulmonary hypertension of the newborn. Paediatr. Respir. Rev. sixteen, 157–161 (2015).
-
Altit, M. et al. Pathophysiology, screening and diagnosis of pulmonary hypertension in infants with bronchopulmonary dysplasia - a review of the literature. Paediatr. Respir. Rev. 23, 16–26 (2017).
-
Nagiub, G., Kanaan, U., Simon, D. & Guglani, 50. Risk factors for development of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review and meta-analysis. Paediatr. Respir. Rev. 23, 27–32 (2017).
-
Al-Ghanem, Grand. et al. Bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis. J. Perinatol. 37, 414–419 (2017).
-
An, H. S. et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ. J. 40, 131–136 (2010).
-
Bhat, R., Salas, A. A., Foster, C., Carlo, West. A. & Ambalavanan, Due north. Prospective assay of pulmonary hypertension in extremely low birth weight infants. Pediatrics 129, e682–e689 (2012).
-
Berenz, A., Vergales, J. E., Swanson, J. R. & Sinkin, R. A. Prove of early pulmonary hypertension is associated with increased mortality in very low nativity weight infants. Am. J. Perinatol. 34, 801–807 (2017).
-
Puthiyachirakkal, M. & Mhanna, Grand. J. Pathophysiology, management, and outcome of persistent pulmonary hypertension of the newborn: a clinical review. Front. Pediatr. 1, 23 (2013).
-
Nair, J. & Lakshminrusimha, S. Update on PPHN: mechanisms and handling. Semin. Perinatol. 38, 78–91 (2014).
-
Steinhorn, R. H. Neonatal pulmonary hypertension. Pediatr. Crit. Intendance Med. xi(2 Suppl.), S79–S84 (2010).
-
Ivy, D. D. et al. Pediatric pulmonary hypertension. J. Am. Coll. Cardiol. 62(25 Suppl.), D117–D126 (2013).
-
Laux, D. et al. Pulmonary hypertension in the preterm infant with chronic lung illness can be acquired by pulmonary vein stenosis: a must-know entity. Pediatr. Cardiol. 37, 313–321 (2016).
-
Mahgoub, L. et al. Pulmonary vein stenosis of ex-premature infants with pulmonary hypertension and bronchopulmonary dysplasia, epidemiology, and survival from a multicenter cohort. Pediatr. Pulmonol. 52, 1063–1070 (2017).
-
Kumar, P., Commission on F, Newborn, American University of P. Use of inhaled nitric oxide in preterm infants. Pediatrics 133, 164–170 (2014).
-
Barrington, K. J., Finer, N. & Pennaforte, T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst. Rev. i, CD000509 (2017).
-
Abman, South. H. et al. Pediatric pulmonary hypertension: guidelines from the American Center Association and American Thoracic Society. Circulation 132, 2037–2099 (2015).
-
Skinner, J. R., Hunter, S. & Hey, Eastward. Due north. Haemodynamic features at presentation in persistent pulmonary hypertension of the newborn and outcome. Arch. Dis. Child. Fetal Neonatal Ed. 74, F26–F32 (1996).
-
Fraisse, A., Geva, T., Gaudart, J. & Wessel, D. L. Doppler echocardiographic predictors of outcome in newborns with persistent pulmonary hypertension. Cardiol. Young 14, 277–283 (2004).
-
Peterson, A. L., Deatsman, S., Frommelt, G. A., Mussatto, M. & Frommelt, P. C. Correlation of echocardiographic markers and therapy in persistent pulmonary hypertension of the newborn. Pediatr. Cardiol. 30, 160–165 (2009).
-
Sehgal, A., Athikarisamy, S. Due east. & Adamopoulos, M. Global myocardial function is compromised in infants with pulmonary hypertension. Acta Paediatr. 101, 410–413 (2012).
-
Aggarwal, Southward. & Natarajan, G. Echocardiographic correlates of persistent pulmonary hypertension of the newborn. Early Hum. Dev. 91, 285–289 (2015).
-
King, M. E., Braun, H., Goldblatt, A., Liberthson, R. & Weyman, A. Due east. Interventricular septal configuration as a predictor of right ventricular systolic hypertension in children: a cross-exclusive echocardiographic study. Apportionment 68, 68–75 (1983).
-
Revanna, M. Grand., Kunjunju, A. & Sehgal, A. Bronchopulmonary dysplasia associated pulmonary hypertension: making the all-time use of bedside echocardiography. Prog. Pediatr. Cardiol. 46, 39–43 (2017).
-
Skinner, J. R. et al. Right middle pressure level decision by Doppler in infants with tricuspid regurgitation. Arch. Dis. Child. 69, 216–220 (1993).
-
Skinner, J. R., Boys, R. J., Heads, A., Hey, E. N. & Hunter, S. Estimation of pulmonary arterial pressure in the newborn: report of the repeatability of four Doppler echocardiographic techniques. Pediatr. Cardiol. 17, 360–369 (1996).
-
Skinner, J. R. in Echocardiography for the Neonatologist (eds Skinner, J. R., Alverson, D. & Hunter, S.) 133–150 (Churchill Livinstone, Edinburgh, 2000).
-
Levy, P. T. et al. Pulmonary artery acceleration time provides a reliable estimate of invasive pulmonary hemodynamics in children. J. Am. Soc. Echocardiogr. 29, 1056–1065 (2016).
-
Levy, P. T. et al. Shape of the right ventricular outflow tract Doppler envelope is a sensitive predictor of altered pulmonary hemodynamics in pediatric patients [Abstract]. J. Am. Coll. Cardiol. 67, 1791 (2016).
-
Abbas, A. E. et al. Noninvasive assessment of pulmonary vascular resistance by Doppler echocardiography. J. Am. Soc. Echocardiogr. 26, 1170–1177 (2013).
-
Pande, A. et al. Non-invasive estimation of pulmonary vascular resistance in patients of pulmonary hypertension in congenital heart disease with unobstructed pulmonary flow. Ann. Pediatr. Cardiol. 7, 92–97 (2014).
-
Xie, Y. et al. Echocardiographic estimation of pulmonary vascular resistance in chronic thromboembolic pulmonary hypertension: utility of correct heart Doppler measurements. Echocardiography 31, 29–33 (2014).
-
Dyer, 1000. et al. Noninvasive Doppler tissue measurement of pulmonary artery compliance in children with pulmonary hypertension. J. Am. Soc. Echocardiogr. 19, 403–412 (2006).
-
Cantinotti, K. et al. Echocardiographic nomograms for chamber diameters and areas in Caucasian children. J. Am. Soc. Echocardiogr. 27, 1279–1292.e2 (2014).
-
Jain, A. et al. A comprehensive echocardiographic protocol for assessing neonatal right ventricular dimensions and function in the transitional period: normative data and z scores. J. Am. Soc. Echocardiogr. 27, 1293–1304 (2014).
-
Koestenberger, M. et al. Reference values and calculation of z-scores of echocardiographic measurements of the normal pediatric correct ventricle. Am. J. Cardiol. 114, 1590–1598 (2014).
-
Koestenberger, M. et al. Echocardiographic reference values for right atrial size in children with and without atrial septal defects or pulmonary hypertension. Pediatr. Cardiol. 37, 686–695 (2016).
-
Sehgal, A., Malikiwi, A., Paul, Eastward., Tan, K. & Menahem, S. A new look at bronchopulmonary dysplasia: postcapillary pathophysiology and cardiac dysfunction. Pulm. Circ. six, 508–515 (2016).
-
Koestenberger, G. et al. Systolic right ventricular part in preterm and term neonates: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 258 patients and calculation of Z-score values. Neonatology 100, 85–92 (2011).
-
Malowitz, J. R. et al. Correct ventricular echocardiographic indices predict poor outcomes in infants with persistent pulmonary hypertension of the newborn. Eur. Heart J. Cardiovasc Imaging 16, 1224–1231 (2015).
-
Levy, P. T. et al. Right ventricular office in preterm and term neonates: reference values for correct ventricle areas and fractional area of modify. J. Am. Soc. Echocardiogr. 28, 559–569 (2015).
-
Mertens, L. et al. Targeted neonatal echocardiography in the neonatal intensive intendance unit: practice guidelines and recommendations for training. Eur. J. Echocardiogr. 12, 715–736 (2011).
-
Czernik, C., Rhode, S., Metze, B., Schmalisch, G. & Buhrer, C. Persistently elevated right ventricular index of myocardial performance in preterm infants with incipient bronchopulmonary dysplasia. PLoS I 7, e38352 (2012).
-
Koestenberger, M. et al. Reference values of tricuspid annular elevation systolic velocity in healthy pediatric patients, adding of z score, and comparison to tricuspid annular plane systolic excursion. Am. J. Cardiol. 109, 116–121 (2012).
-
Aggarwal, S., Stockman, P. T., Klein, One thousand. D. & Natarajan, G. The right ventricular systolic to diastolic duration ratio: a simple prognostic marker in congenital diaphragmatic hernia? Acta Paediatr. 100, 1315–1318 (2011).
-
Patel, North., Mills, J. F. & Cheung, 1000. One thousand. Assessment of right ventricular function using tissue Doppler imaging in infants with pulmonary hypertension. Neonatology 96, 193–199 (2009).
-
Moenkemeyer, F. & Patel, N. Right ventricular diastolic office measured by tissue Doppler imaging predicts early on issue in congenital diaphragmatic hernia. Pediatr. Crit. Intendance Med. 15, 49–55 (2014).
-
James, A. T., Corcoran, J. D., McNamara, P. J., Franklin, O. & El-Khuffash, A. F. The effect of milrinone on correct and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiol. Young 26, 1–10 (2015).
-
Levy, P. T., Holland, M. R., Sekarski, T. J., Hamvas, A. & Singh, K. One thousand. Feasibility and reproducibility of systolic right ventricular strain measurement by speckle-tracking echocardiography in premature infants. J. Am. Soc. Echocardiogr. 26, 1201–1213 (2013).
-
Schubert, U., Muller, M., Abdul-Khaliq, H. & Norman, M. Preterm nativity is associated with altered myocardial function in infancy. J. Am. Soc. Echocardiogr. 29, 670–678 (2016).
-
Jain, A. et al. Comprehensive evaluation of cardiac function and haemodynamics using conventional tissue Doppler and speckle tracking echocardiography in neonates with persistent pulmonary hypertension of the newborn [Abstruse]. Eur. J. Pediatr. 175, 1464–1465 (2016).
-
Levy, P. T. et al. Maturational patterns of systolic ventricular deformation mechanics by ii-dimensional speckle-tracking echocardiography in preterm infants over the first yr of age. J. Am. Soc. Echocardiogr. 30, 685–698.e1 (2017).
-
Sehgal, A., Ibrahim, Thousand. & Tan, Thou. Cardiac function and its development with pulmonary vasodilator therapy: a myocardial deformation written report. Echocardiography 31, E185–E188 (2014).
Acknowledgements
All members of the European Special Interest Group 'Neonatologist Performed Echocardiography' are listed in the appendix. All these members take substantially contributed to the conception and revision of the manuscript and approved the final version to be published. Fiscal back up of publication costs by the European Club for Paediatric Research (ESPR) is gratefully acknowledged.
Writer information
Affiliations
Consortia
Respective writer
Ethics declarations
Competing interests
A.E.K. is in receipt of an Irish Health Research Lath Clinical Trials Network Grant (HRB CTN 2014-ten) and an European union FP7/2007-2013 grant (agreement no. 260777, The HIP Trial). A.Thou. owned equity in Neonatal Echo Skills and has received grant support from the American Heart Association. D.5.L. is in receipt of an EU FP7/2007-2013 (agreement no. 260777 the HIP trial). E.D. received lecture fees and consulting fees from Chiesi Pharmaceutical. Due east.Due north. received grant support from Research Council of Norway and Vestfold Hospital Trust. Chiliad.B. received lecture fees from Chiesi Pharmaceutical. M.B. holds a patent, "Thermal shield for the newborn baby". South.G. received grant support from National Found of Health Research, Health Technology Assessment (xi/92/xv), Britain. S.R. received lecture fees for Phillips Ultrasound and GE Ultrasound. W.P.B. has received grant support from The Netherlands Organization for Health and Development (ZonMw; grant numbers 843002622 and 843002608). Z.One thousand. has received lecture fees from Chiesi Pharmaceutical. The remaining authors alleged no competing interests.
Additional data
Publisher'southward notation: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the European Special Interest Group 'Neonatologist Performed Echocardiography' (NPE), endorsed by the European Society for Paediatric Enquiry (ESPR) and European Board of Neonatology (EBN) are listed in the Appendix.
APPENDIX
APPENDIX
European Special Interest Group 'Neonatologist Performed Echocardiography' (NPE), endorsed by the European Society for Paediatric Research (ESPR) and European Board of Neonatology (EBN)
de Boode W. P. (chairman), Department of Neonatology, Radboud University Medical Center, Radboud Institute for Health Sciences, Amalia Children's Hospital, Nijmegen, Holland (willem.deboode@radboudumc.nl)
Austin T., Section of Neonatology, Rosie Infirmary, Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom (topun.austin@addenbrookes.nhs.uk)
Bohlin K., Department of Neonatology, Karolinska Academy Hospital, Karolinska Institutet, Stockholm, Sweden (kajsa.bohlin@ki.se)
Bravo M. C., Department of Neonatology, La Paz University Hospital, Madrid, Spain (mcarmen.bravo@salud.madrid.org)
Breatnach C. R., Department of Neonatology, The Rotunda Hospital, Dublin, Ireland (colm.breatnach@gmail.com)
Breindahl M., Karolinska Academy Infirmary, Karolinska Institutet, Stockholm, Sweden (morten.breindahl@sll.se)
Dempsey E, INFANT Middle, Cork University Maternity Hospital, University College Cork, Republic of ireland (thou.dempsey@ucc.ie)
El-Khuffash A., Department of Neonatology, The Rotunda Hospital, Dublin, Republic of ireland; Department of Pediatrics, The Imperial College of Surgeons in Ireland, Dublin, Ireland (afifelkhuffash@rcsi.ie)
Groves A. M., Sectionalisation of Newborn Medicine, Mount Sinai Kravis Children's Hospital, New York, NY, USA (alan.groves@mssm.edu)
Gupta Southward., University Hospital of North Tees, Durham University, Stockton-on-Tees, U.k. (samir.gupta@nth.nhs.uk)
Horsberg Eriksen B., Department of Pediatrics, Møre and Romsdal Hospital Trust, Ålesund, Norway; (beate.eriksen@me.com)
Levy P. T., Department of Pediatrics, Washington Academy School of Medicine, Saint Louis, MO, Us; Department of Pediatrics, Goryeb Children's Hospital, Morristown, NJ, USA (Levy_P@kids.wustl.edu)
McNamara P. J., Departments of Pediatrics and Physiology, University of Toronto, Toronto, ON, Canada (patrick.mcnamara@sickkids.ca)
Molnar Z., John Radcliffe Infirmary, Oxford, United Kingdom (zoltan.Molnar@ouh.nhs.uk)
Nestaas E., Constitute of Clinical Medicine, Faculty of Medicine, Academy of Oslo, Kingdom of norway; Department of Cardiology and Center for Cardiological Innovation, Oslo University Hospital, Rikshospitalet, Oslo, Norway; Department of Paediatrics, Vestfold Hospital Trust, Tønsberg, Norway (nestaas@hotmail.com)
Rogerson S. R., The Royal Women'south Infirmary, Parkville, VIC, Australia (sheryle.Rogerson@thewomens.org.au)
Roehr C. C., Department of Paediatrics, Academy of Oxford, John Radcliffe Hospital, Oxford, United Kingdom (charles.roehr@paediatrics.ox.ac.uk)
Savoia M., Azienda Ospedaliero-Universitaria S. Maria della Misericordia, Udine, Italia (marilena.savoia@gmail.com)
Schubert U., Section of Clinical Science, Intervention and Engineering science, Karolinska Institutet, Stockholm, Sweden (ulfschubert@gmx.de)
Schwarz C. E., Section of Neonatology, University Children's Hospital of Tübingen, Tübingen, Germany (c.schwarz@med.uni-tuebingen.de)
Sehgal A., Department of Pediatrics, Monash Academy, Melbourne, Commonwealth of australia (arvind.sehgal@monash.edu)
Singh Y., Addenbrooke's Infirmary, Cambridge University Hospitals NHS Foundation Trust, Cambridge, Uk (yogen.Singh@nhs.net)
Slieker M. M., Section of Paediatric Cardiology, Radboudumc Amalia Children'due south Hospital, Nijmegen, The Netherlands (Martijn.Slieker@radboudumc.nl)
Tissot C., Department of Pediatrics, Clinique des Grangettes, Chêne Bougeries, Switzerland (cecile.tissot@hotmail.com)
van der Lee R., Department of Neonatology, Radboud University Medical Center, Radboud Institute for Health Sciences, Amalia Children'due south Hospital, Nijmegen, Holland (Robin.vanderLee@radboudumc.nl)
van Laere D., Section of Pediatrics, Antwerp University Hospital UZA, Edegem, Belgium (david.VanLaere@uza.be)
van Overmeire B., Department of Neonatology, Academy Hospital Brussels, Brussels, Belgium (bart.van.overmeire@erasme.ulb.ac.be)
van Wyk L., Department of Paediatrics & Child Health, Academy of Stellenbosch, Greatcoat Town, South Africa (lizelle@sun.air conditioning.za)
Rights and permissions
Open up Access This article is licensed nether a Creative Eatables Attribution four.0 International License, which permits use, sharing, accommodation, distribution and reproduction in any medium or format, as long as you requite advisable credit to the original author(s) and the source, provide a link to the Creative Commons license, and bespeak if changes were made. The images or other third party material in this commodity are included in the commodity'due south Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the commodity's Creative Eatables license and your intended utilize is not permitted by statutory regulation or exceeds the permitted use, you will demand to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/four.0/.
Reprints and Permissions
Virtually this article
Cite this commodity
de Boode, West.P., Singh, Y., Molnar, Z. et al. Awarding of Neonatologist Performed Echocardiography in the cess and management of persistent pulmonary hypertension of the newborn. Pediatr Res 84, 68–77 (2018). https://doi.org/x.1038/s41390-018-0082-0
-
Published:
-
Consequence Date:
-
DOI : https://doi.org/ten.1038/s41390-018-0082-0
Further reading
Source: https://www.nature.com/articles/s41390-018-0082-0
0 Response to "What Are the Two Most Common Findings on Premature Baby Echocardiogram"
Post a Comment